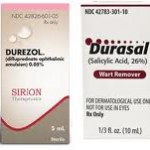
The FDA is taking time to warn pharmacists about potential dispensing errors that could occur as a result of a mix-up between 2 drugs with very similar brand names. Confusion over the two medications could cause patients to be given the wrong prescription and subsequently endure serious injuries. The two brands in question are Durezol [...]