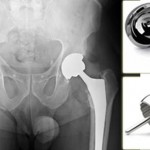
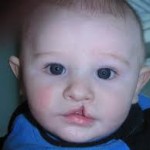
The FDA (Food and Drug Administration) “stands accused of being little more than a rubber-stamping agency for Big Pharma.” “The FDA has progressively morphed into a mere pawn and instrument of the drug industry, which has little to do with drug safety, and everything to do with maximizing profits.” writes Dr. Mercola in an article, [...]