 Did Johnson & Johnson’s directors ignore wrongdoing?
Did Johnson & Johnson’s directors ignore wrongdoing?
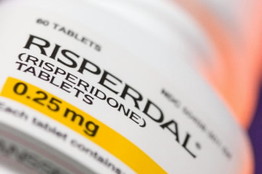
Johnson & Johnson was sued by investors in 2010 regarding the company’s marketing practices for Risperdal and for the production miscues that led to recalls of products such as children’s Tylenol.
As part of a settlement of claims filed against Johnson & Johnson’s directors in federal court in Trenton, New Jersey. J&J officials agreed to create a board-level group to oversee subsidiaries’ compliance with regulatory rules and to adopt updated risk-management policies .
Attorneys for Johnson & Jonson investors stated in court papers that the above changes will force directors to “take responsibility for identifying and addressing problems” before they are out of control.
One of the changes requires the boards new Regulatory, Compliance and Government Affairs Committee, which must consist solely of independent directors, to “provide an annual report to J&J shareholders” about the units’ regulatory compliance efforts, according to the filing.
Any recovery under derivative suits filed by Investors’ of Johnson & Johnson against the drug maker, is returned to the company’s coffers rather than distributed to shareholders
Johnson & Johnson’s company officials have said that J&J is in “talks” to resolve investigations of the marketing of its Risperdal anti-psychotic drug and other medications. J&J has reportedly agreed to pay $2.2 billion to settle. J&J spokesman, Al Wasilewski said in an email that “Johnson and Johnson has a long commitment to compliance, quality and good corporate governance” and “continues to deny the claims”. Although J&J has agreed to the settlement to “eliminate the burden” of further litigation.
For more information and a Free case review contact The Maher Law Firm or Frank M. Eidson



