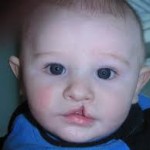
HIV Drugs Side Effects HealthDay (1/31, Dallas) reports, “– Although there remains a possibility that some of these medications might cause birth defects, such as cleft lip and palate,” according to a study published in the January issue of Cleft Palate–Craniofacial Journal. To analyze the possible association between antiretroviral drugs and birth defects,Vassiliki Cartsos, an associate [...]