 As of March 2013, NuvaRing litigation filed in state and federal courts are comprised of over 1,250 individual lawsuits
As of March 2013, NuvaRing litigation filed in state and federal courts are comprised of over 1,250 individual lawsuits
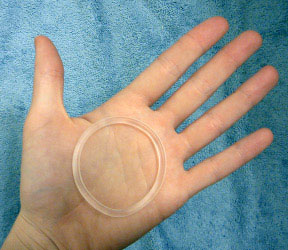
NuvaRing is birth control in the form of a non-oral hormonal contraception that is placed in the vagina once a month to prevent pregnancy. NuvaRing contains estrogen and progestin which thickens the cervical mucus and keeps the ovaries from producing mature eggs.
The accusations against Merck (formerly Schering-Plough, formerly Organon), the manufacturer of NuvaRing, are failure to adequately research the side effects of NuvaRing, or to sufficiently warn woman of the possibility of an increased risk of blood clots.
NuvaRing Side Effects:
Possible Serious Side Effects of Non Oral Hormonal Contraception:
- Heart Attack
- Stroke
- Pulmonary Embolism, blood Clots in the legs, lungs, heart or brain
- Vaginal Infections, Irritations and/or Secretion
- Headaches
The first trial will begin late October 2013, according to an order issued in March 2013. The lawsuit involves a woman who suffered a pulmonary embolism while using NuvaRing birth control. Nine other cases have been chosen for “bellwether” cases (cases that help predict how juries may respond) beginning in May of 2013.
As a consumer and a patient, you have the right to trust your medication is safe. Tragically, this is not always the case. When a pharmaceutical company acts negligently, its actions can result in serious side effects. Victims, however, may recoup damages through a successful product liability claim against the drug’s maker.
Well versed in the product liability laws that protect consumers, our NuvaRing attorneys have handled countless lawsuits involving harmful pharmaceuticals. If you have experienced NuvaRing side effects, contact us today and discuss your potential claim with an experienced Maher Law Firm trial attorney.



