 Class 1 recall has been called on 4 buretrol solution sets
Class 1 recall has been called on 4 buretrol solution sets
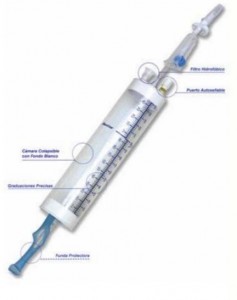
Baxter Healthcare, the manufacturer of Buretrol solution sets, initiated a recall of the product on September 7th 2012, due to a potential ball-valve malfunction.
Buretrol Solution set and Clearlink System Buretrol Solution set are part of an interlink system. Each system contains a ball-valve that may be putting patients at risk of an air embolism.
The ball-valve may allow air to flow past the valve and enter the products’ tubing with pre-measured fluids, causing an air embolism.
The FDA (Food and Drug Administration) raised the recall of four Buretrol Solution sets to class 1 due to the ball-valve malfunction.
The recalled sets were manufactured from April 30, 2003 to July 26, 2012 and were distributed from May 1, 2003 to August 16, 2012.
The recalled units include all lots of the solution sets with 150 mL Burettes labeled with or without DEHP, and include product codes:
2C7519
2H7519
2C8819
2H8819
If you believe that you or a loved one has suffered injury or death due to a defective or dangerous pharmaceutical, you may have the right to compensation for your injuries. The Maher Law Firm and Frank M. Eidson would like to help. We invite you to contact our trial tested Lawyers today.



