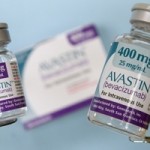
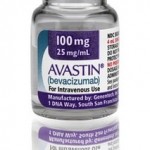
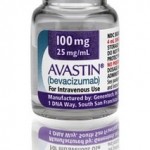
The U.S. company, Medical Device King (also known as Pharmalogical) has distributed a cancer medicine that was not approved by the FDA (Food and Drug Administration) FDA lab tests confirm that at least one batch of a counterfeit version of Roche’s Altuzan (bevacizumab) contains no active ingredient. Aluzan, an injectable cancer medicine, is not approved [...]