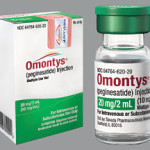
Affymax and Takeda, the manufacturers of Omontys (Peginesatide) are issuing a voluntary recall of the drug due to life threatening and fatal events reported in patients receiving Omontys. This voluntary recall is being conducted with the knowledge of the FDA (Food and Drug Administration), who approved the drug in March of 2012. In a statement, [...]